| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2022-03-07 02:49:08 UTC |
|---|
| HMDB ID | HMDB0001060 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Ubiquinol 8 |
|---|
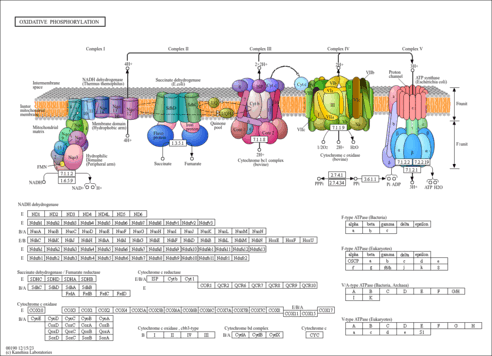
| Description | Ubiquinol 8 is a ubiquinol in which the polyprenyl substituent is octaprenyl. Ubiquinol-8 is the reduced form of ubiquinone-8. Ubiquinone (also known as coenzyme Q) is an isoprenoid quinone that functions as an electron carrier in membranes. In eukaryotes, ubiquinone is found mostly within the inner mitochondrial membrane where it functions in respiratory electron transport, transferring two electrons from either complex I (NADH dehydrogenase) or complex II (succinate-ubiquinone reductase) to complex III (bc1 complex). The quinone nucleus of ubiquinone is derived directly from 4-hydroxybenzoate, while the isoprenoid subunits of the polyisoprenoid tail are synthesized via the methylerythritol phosphate pathway, which feeds isoprene units into the polyprenyl biosynthesis pathways. The number of isoprenoid subunits in the ubiquinone side chain varies in different species. For example, Saccharomyces cerevisiae has 6 such subunits, Escherichia coli K-12 has 8, rat and mouse have 9, and Homo sapiens has 10. Ubiquinol-8 is effective as an anti-oxidant. By donating one of its hydrogen atoms to become the free-radical semiquinone (.Q-), it can neutralize a lipid peroxyl radical. The free-radical semiquinone is then restored to a non-free-radical state by the respiratory chain Q cycle. Ubiquinol or the free-radical semiquinone can also regenerate the Vitamin E tocopheroxyl radical by electron donation (http://www.benbest.com/nutrceut/CoEnzymeQ.html). |
|---|
| Structure | COC1=C(OC)C(O)=C(C\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CCC=C(C)C)C(C)=C1O InChI=1S/C49H76O4/c1-36(2)20-13-21-37(3)22-14-23-38(4)24-15-25-39(5)26-16-27-40(6)28-17-29-41(7)30-18-31-42(8)32-19-33-43(9)34-35-45-44(10)46(50)48(52-11)49(53-12)47(45)51/h20,22,24,26,28,30,32,34,50-51H,13-19,21,23,25,27,29,31,33,35H2,1-12H3/b37-22+,38-24+,39-26+,40-28+,41-30+,42-32+,43-34+ |
|---|
| Synonyms | | Value | Source |
|---|
| Reduced coenzyme Q8 | ChEBI | | Ubiquinol(8) | ChEBI | | Ubiquinol-8 | HMDB |
|
|---|
| Chemical Formula | C49H76O4 |
|---|
| Average Molecular Weight | 729.1253 |
|---|
| Monoisotopic Molecular Weight | 728.57436092 |
|---|
| IUPAC Name | 2,3-dimethoxy-5-methyl-6-[(2E,6E,10E,14E,18E,22E,26E)-3,7,11,15,19,23,27,31-octamethyldotriaconta-2,6,10,14,18,22,26,30-octaen-1-yl]benzene-1,4-diol |
|---|
| Traditional Name | ubiquinol-8 |
|---|
| CAS Registry Number | 74075-00-6 |
|---|
| SMILES | COC1=C(OC)C(O)=C(C\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CCC=C(C)C)C(C)=C1O |
|---|
| InChI Identifier | InChI=1S/C49H76O4/c1-36(2)20-13-21-37(3)22-14-23-38(4)24-15-25-39(5)26-16-27-40(6)28-17-29-41(7)30-18-31-42(8)32-19-33-43(9)34-35-45-44(10)46(50)48(52-11)49(53-12)47(45)51/h20,22,24,26,28,30,32,34,50-51H,13-19,21,23,25,27,29,31,33,35H2,1-12H3/b37-22+,38-24+,39-26+,40-28+,41-30+,42-32+,43-34+ |
|---|
| InChI Key | LOJUQFSPYHMHEO-SGHXUWJISA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as polyprenyl quinols. Polyprenyl quinols are compounds containing a polyisoprene chain attached to a quinol(hydroquinone) at the second ring position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Quinone and hydroquinone lipids |
|---|
| Direct Parent | Polyprenyl quinols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetraterpenoid
- 2-polyprenyl-6-methoxyphenol
- Polyprenylbenzoquinol
- Polyprenylphenol
- Ubiquinol skeleton
- Methoxyphenol
- O-dimethoxybenzene
- Dimethoxybenzene
- Anisole
- Hydroquinone
- M-cresol
- Phenoxy compound
- O-cresol
- Phenol ether
- Methoxybenzene
- Alkyl aryl ether
- Phenol
- Toluene
- Monocyclic benzene moiety
- Benzenoid
- Ether
- Organooxygen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Not Available |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatized |
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - Ubiquinol 8 GC-MS (TMS_1_1) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ubiquinol 8 GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ubiquinol 8 GC-MS (TMS_2_1) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ubiquinol 8 GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Ubiquinol 8 GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-10-18 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ubiquinol 8 10V, Positive-QTOF | splash10-004i-0323324900-8d5262afd36dcbc7148a | 2019-02-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ubiquinol 8 20V, Positive-QTOF | splash10-0032-1648692100-f4b35e5836e1b0685dbe | 2019-02-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ubiquinol 8 40V, Positive-QTOF | splash10-0532-5487958100-34e9af02e9a7005e70a4 | 2019-02-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ubiquinol 8 10V, Negative-QTOF | splash10-004i-0000000900-456744203daa0fca5d76 | 2019-02-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ubiquinol 8 20V, Negative-QTOF | splash10-0h00-0000009500-add178adb113c4e8faa3 | 2019-02-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ubiquinol 8 40V, Negative-QTOF | splash10-03k9-3000019100-471dfb54d9e8cb8939f8 | 2019-02-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ubiquinol 8 10V, Positive-QTOF | splash10-057j-6405569600-34b9f4abc22e1814c315 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ubiquinol 8 20V, Positive-QTOF | splash10-000t-4904532000-2ec1e0341ea818671a91 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ubiquinol 8 40V, Positive-QTOF | splash10-0036-9613310000-3825f8084c00c72645ec | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ubiquinol 8 10V, Negative-QTOF | splash10-004i-0000000900-753eb68b8e8835d49d63 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ubiquinol 8 20V, Negative-QTOF | splash10-00mk-0910108500-1324247480f23e6031ea | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Ubiquinol 8 40V, Negative-QTOF | splash10-001i-0942167000-3f62ea4336556f2f28ce | 2021-09-22 | Wishart Lab | View Spectrum |
|
|---|
| General References | - Pastore A, Giovamberardino GD, Bertini E, Tozzi G, Gaeta LM, Federici G, Piemonte F: Simultaneous determination of ubiquinol and ubiquinone in skeletal muscle of pediatric patients. Anal Biochem. 2005 Jul 15;342(2):352-5. Epub 2005 Mar 7. [PubMed:15989930 ]
- Maneiro E, Lopez-Armada MJ, de Andres MC, Carames B, Martin MA, Bonilla A, Del Hoyo P, Galdo F, Arenas J, Blanco FJ: Effect of nitric oxide on mitochondrial respiratory activity of human articular chondrocytes. Ann Rheum Dis. 2005 Mar;64(3):388-95. [PubMed:15708893 ]
- Nohl H, Gille L, Staniek K: Intracellular generation of reactive oxygen species by mitochondria. Biochem Pharmacol. 2005 Mar 1;69(5):719-23. Epub 2005 Jan 20. [PubMed:15710349 ]
|
|---|