| Record Information |
|---|
| Version | 5.0 |
|---|
| Status | Detected and Quantified |
|---|
| Creation Date | 2005-11-16 15:48:42 UTC |
|---|
| Update Date | 2023-02-21 17:14:30 UTC |
|---|
| HMDB ID | HMDB0000078 |
|---|
| Secondary Accession Numbers | - HMDB00078
- HMDB0028775
- HMDB28775
|
|---|
| Metabolite Identification |
|---|
| Common Name | Cysteinylglycine |
|---|
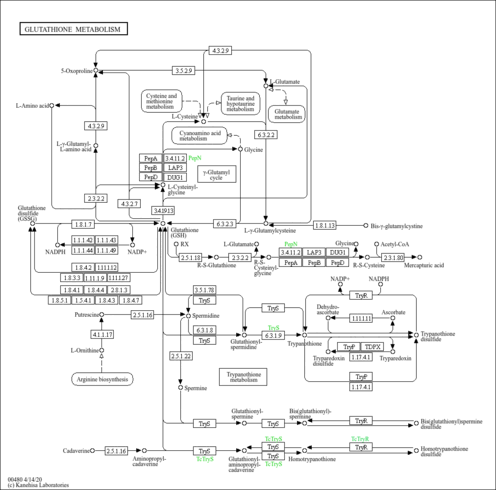
| Description | Cysteinylglycine is a naturally occurring dipeptide composed of cysteine and glycine. It is derived from the breakdown of glutathione (a tripeptide). In plasma, cysteinylglycine is in a reduced, oxidized, and protein-bound form (aminothiol) and interacts via redox and disulphide exchange reactions in a dynamic system referred to as redox thiol status (PMID: 8642471 ). Spermatozoa of sub-fertile men contain significantly higher thiol concentrations as compared with those of fertile men. The detrimental effect on embryo quality of a high homocysteine (Hcy) concentration in the ejaculate and in the follicular fluid is intriguing and may suggest that Hcy is inversely associated with fertility outcome (PMID: 16556671 ). Rheumatoid arthritis (RA) is a chronic inflammatory disease which involves the synovial membrane of multiple diarthroidal joints causing damage to cartilage and bones. The damage process seems to be related to an overproduction of oxygen reactive species inducing an oxidative perturbation with an increase in some oxidized forms (disulfides and protein mixed disulfides) and a decrease in free thiols (PMID: 15895891 ). Imipenem (thienamycin formamidine) is a broad-spectrum beta-lactam antibiotic, always used in combination with cilastatin in order to avoid the premature breakdown of imipenem by renal tubular dipeptidase. As this dipeptidase also hydrolyzes the glutathione metabolite cysteinylglycine, the therapeutic association of imipenem and cilastatin causes plasma levels of cysteinylglycine to increase significantly, while cysteine levels are decreased and homocysteine levels are unaffected. Therefore, antibiotic treatment using imipenem-cilastatin induces important metabolic changes that should not remain unrecognized (PMID: 15843241 ). |
|---|
| Structure | InChI=1S/C5H10N2O3S/c6-3(2-11)5(10)7-1-4(8)9/h3,11H,1-2,6H2,(H,7,10)(H,8,9)/t3-/m0/s1 |
|---|
| Synonyms | | Value | Source |
|---|
| CG | ChEBI | | Cys-gly | ChEBI | | N-L-Cysteinylglycine | ChEBI | | L-Cysteinylglycine | Kegg | | N-Cysteinyl glycine | HMDB | | N-Cysteinyl-glycine | HMDB | | N-L-Cysteinyl-glycine | HMDB | | Cysteinyl-glycine | HMDB | | C-g Dipeptide | HMDB | | CG Dipeptide | HMDB | | Cysteine glycine dipeptide | HMDB | | Cysteine-glycine dipeptide | HMDB | | L-Cys-gly | HMDB | | N-Cysteinylglycine | HMDB | | Cysteinylglycine | ChEBI |
|
|---|
| Chemical Formula | C5H10N2O3S |
|---|
| Average Molecular Weight | 178.21 |
|---|
| Monoisotopic Molecular Weight | 178.041212886 |
|---|
| IUPAC Name | 2-[(2R)-2-amino-3-sulfanylpropanamido]acetic acid |
|---|
| Traditional Name | Cys-Gly |
|---|
| CAS Registry Number | 19246-18-5 |
|---|
| SMILES | N[C@@H](CS)C(=O)NCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C5H10N2O3S/c6-3(2-11)5(10)7-1-4(8)9/h3,11H,1-2,6H2,(H,7,10)(H,8,9)/t3-/m0/s1 |
|---|
| InChI Key | ZUKPVRWZDMRIEO-VKHMYHEASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- Cysteine or derivatives
- Alpha-amino acid or derivatives
- Amino acid or derivatives
- Amino acid
- Carboxamide group
- Secondary carboxylic acid amide
- Alkylthiol
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Organosulfur compound
- Primary aliphatic amine
- Primary amine
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Amine
- Organopnictogen compound
- Organic oxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Physiological effect | Not Available |
|---|
| Disposition | |
|---|
| Process | |
|---|
| Role | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Molecular Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Experimental Chromatographic Properties | Experimental Collision Cross Sections |
|---|
| Predicted Molecular Properties | |
|---|
| Predicted Chromatographic Properties | Predicted Collision Cross SectionsPredicted Kovats Retention IndicesUnderivatizedDerivatized| Derivative Name / Structure | SMILES | Kovats RI Value | Column Type | Reference |
|---|
| Cysteinylglycine,1TMS,isomer #1 | C[Si](C)(C)OC(=O)CNC(=O)[C@@H](N)CS | 1830.5 | Semi standard non polar | 33892256 | | Cysteinylglycine,1TMS,isomer #2 | C[Si](C)(C)SC[C@H](N)C(=O)NCC(=O)O | 1900.0 | Semi standard non polar | 33892256 | | Cysteinylglycine,1TMS,isomer #3 | C[Si](C)(C)N[C@@H](CS)C(=O)NCC(=O)O | 1841.7 | Semi standard non polar | 33892256 | | Cysteinylglycine,1TMS,isomer #4 | C[Si](C)(C)N(CC(=O)O)C(=O)[C@@H](N)CS | 1828.5 | Semi standard non polar | 33892256 | | Cysteinylglycine,2TMS,isomer #1 | C[Si](C)(C)OC(=O)CNC(=O)[C@@H](N)CS[Si](C)(C)C | 1972.1 | Semi standard non polar | 33892256 | | Cysteinylglycine,2TMS,isomer #1 | C[Si](C)(C)OC(=O)CNC(=O)[C@@H](N)CS[Si](C)(C)C | 1895.4 | Standard non polar | 33892256 | | Cysteinylglycine,2TMS,isomer #1 | C[Si](C)(C)OC(=O)CNC(=O)[C@@H](N)CS[Si](C)(C)C | 3225.3 | Standard polar | 33892256 | | Cysteinylglycine,2TMS,isomer #2 | C[Si](C)(C)N[C@@H](CS)C(=O)NCC(=O)O[Si](C)(C)C | 1917.4 | Semi standard non polar | 33892256 | | Cysteinylglycine,2TMS,isomer #2 | C[Si](C)(C)N[C@@H](CS)C(=O)NCC(=O)O[Si](C)(C)C | 1805.8 | Standard non polar | 33892256 | | Cysteinylglycine,2TMS,isomer #2 | C[Si](C)(C)N[C@@H](CS)C(=O)NCC(=O)O[Si](C)(C)C | 2440.2 | Standard polar | 33892256 | | Cysteinylglycine,2TMS,isomer #3 | C[Si](C)(C)OC(=O)CN(C(=O)[C@@H](N)CS)[Si](C)(C)C | 1807.6 | Semi standard non polar | 33892256 | | Cysteinylglycine,2TMS,isomer #3 | C[Si](C)(C)OC(=O)CN(C(=O)[C@@H](N)CS)[Si](C)(C)C | 1732.1 | Standard non polar | 33892256 | | Cysteinylglycine,2TMS,isomer #3 | C[Si](C)(C)OC(=O)CN(C(=O)[C@@H](N)CS)[Si](C)(C)C | 2749.2 | Standard polar | 33892256 | | Cysteinylglycine,2TMS,isomer #4 | C[Si](C)(C)N[C@@H](CS[Si](C)(C)C)C(=O)NCC(=O)O | 1965.5 | Semi standard non polar | 33892256 | | Cysteinylglycine,2TMS,isomer #4 | C[Si](C)(C)N[C@@H](CS[Si](C)(C)C)C(=O)NCC(=O)O | 1930.5 | Standard non polar | 33892256 | | Cysteinylglycine,2TMS,isomer #4 | C[Si](C)(C)N[C@@H](CS[Si](C)(C)C)C(=O)NCC(=O)O | 2897.7 | Standard polar | 33892256 | | Cysteinylglycine,2TMS,isomer #5 | C[Si](C)(C)SC[C@H](N)C(=O)N(CC(=O)O)[Si](C)(C)C | 1927.6 | Semi standard non polar | 33892256 | | Cysteinylglycine,2TMS,isomer #5 | C[Si](C)(C)SC[C@H](N)C(=O)N(CC(=O)O)[Si](C)(C)C | 1967.0 | Standard non polar | 33892256 | | Cysteinylglycine,2TMS,isomer #5 | C[Si](C)(C)SC[C@H](N)C(=O)N(CC(=O)O)[Si](C)(C)C | 2922.2 | Standard polar | 33892256 | | Cysteinylglycine,2TMS,isomer #6 | C[Si](C)(C)N([C@@H](CS)C(=O)NCC(=O)O)[Si](C)(C)C | 1995.5 | Semi standard non polar | 33892256 | | Cysteinylglycine,2TMS,isomer #6 | C[Si](C)(C)N([C@@H](CS)C(=O)NCC(=O)O)[Si](C)(C)C | 1850.8 | Standard non polar | 33892256 | | Cysteinylglycine,2TMS,isomer #6 | C[Si](C)(C)N([C@@H](CS)C(=O)NCC(=O)O)[Si](C)(C)C | 2636.0 | Standard polar | 33892256 | | Cysteinylglycine,2TMS,isomer #7 | C[Si](C)(C)N[C@@H](CS)C(=O)N(CC(=O)O)[Si](C)(C)C | 1906.1 | Semi standard non polar | 33892256 | | Cysteinylglycine,2TMS,isomer #7 | C[Si](C)(C)N[C@@H](CS)C(=O)N(CC(=O)O)[Si](C)(C)C | 1784.4 | Standard non polar | 33892256 | | Cysteinylglycine,2TMS,isomer #7 | C[Si](C)(C)N[C@@H](CS)C(=O)N(CC(=O)O)[Si](C)(C)C | 2461.6 | Standard polar | 33892256 | | Cysteinylglycine,3TMS,isomer #1 | C[Si](C)(C)N[C@@H](CS[Si](C)(C)C)C(=O)NCC(=O)O[Si](C)(C)C | 2013.7 | Semi standard non polar | 33892256 | | Cysteinylglycine,3TMS,isomer #1 | C[Si](C)(C)N[C@@H](CS[Si](C)(C)C)C(=O)NCC(=O)O[Si](C)(C)C | 2011.9 | Standard non polar | 33892256 | | Cysteinylglycine,3TMS,isomer #1 | C[Si](C)(C)N[C@@H](CS[Si](C)(C)C)C(=O)NCC(=O)O[Si](C)(C)C | 2535.2 | Standard polar | 33892256 | | Cysteinylglycine,3TMS,isomer #2 | C[Si](C)(C)OC(=O)CN(C(=O)[C@@H](N)CS[Si](C)(C)C)[Si](C)(C)C | 1945.7 | Semi standard non polar | 33892256 | | Cysteinylglycine,3TMS,isomer #2 | C[Si](C)(C)OC(=O)CN(C(=O)[C@@H](N)CS[Si](C)(C)C)[Si](C)(C)C | 2020.3 | Standard non polar | 33892256 | | Cysteinylglycine,3TMS,isomer #2 | C[Si](C)(C)OC(=O)CN(C(=O)[C@@H](N)CS[Si](C)(C)C)[Si](C)(C)C | 2737.4 | Standard polar | 33892256 | | Cysteinylglycine,3TMS,isomer #3 | C[Si](C)(C)OC(=O)CNC(=O)[C@H](CS)N([Si](C)(C)C)[Si](C)(C)C | 2043.2 | Semi standard non polar | 33892256 | | Cysteinylglycine,3TMS,isomer #3 | C[Si](C)(C)OC(=O)CNC(=O)[C@H](CS)N([Si](C)(C)C)[Si](C)(C)C | 2006.3 | Standard non polar | 33892256 | | Cysteinylglycine,3TMS,isomer #3 | C[Si](C)(C)OC(=O)CNC(=O)[C@H](CS)N([Si](C)(C)C)[Si](C)(C)C | 2330.6 | Standard polar | 33892256 | | Cysteinylglycine,3TMS,isomer #4 | C[Si](C)(C)N[C@@H](CS)C(=O)N(CC(=O)O[Si](C)(C)C)[Si](C)(C)C | 1898.6 | Semi standard non polar | 33892256 | | Cysteinylglycine,3TMS,isomer #4 | C[Si](C)(C)N[C@@H](CS)C(=O)N(CC(=O)O[Si](C)(C)C)[Si](C)(C)C | 1895.9 | Standard non polar | 33892256 | | Cysteinylglycine,3TMS,isomer #4 | C[Si](C)(C)N[C@@H](CS)C(=O)N(CC(=O)O[Si](C)(C)C)[Si](C)(C)C | 2297.6 | Standard polar | 33892256 | | Cysteinylglycine,3TMS,isomer #5 | C[Si](C)(C)SC[C@@H](C(=O)NCC(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 2108.0 | Semi standard non polar | 33892256 | | Cysteinylglycine,3TMS,isomer #5 | C[Si](C)(C)SC[C@@H](C(=O)NCC(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 2061.4 | Standard non polar | 33892256 | | Cysteinylglycine,3TMS,isomer #5 | C[Si](C)(C)SC[C@@H](C(=O)NCC(=O)O)N([Si](C)(C)C)[Si](C)(C)C | 2608.2 | Standard polar | 33892256 | | Cysteinylglycine,3TMS,isomer #6 | C[Si](C)(C)N[C@@H](CS[Si](C)(C)C)C(=O)N(CC(=O)O)[Si](C)(C)C | 1993.4 | Semi standard non polar | 33892256 | | Cysteinylglycine,3TMS,isomer #6 | C[Si](C)(C)N[C@@H](CS[Si](C)(C)C)C(=O)N(CC(=O)O)[Si](C)(C)C | 2050.4 | Standard non polar | 33892256 | | Cysteinylglycine,3TMS,isomer #6 | C[Si](C)(C)N[C@@H](CS[Si](C)(C)C)C(=O)N(CC(=O)O)[Si](C)(C)C | 2462.8 | Standard polar | 33892256 | | Cysteinylglycine,3TMS,isomer #7 | C[Si](C)(C)N(CC(=O)O)C(=O)[C@H](CS)N([Si](C)(C)C)[Si](C)(C)C | 2018.3 | Semi standard non polar | 33892256 | | Cysteinylglycine,3TMS,isomer #7 | C[Si](C)(C)N(CC(=O)O)C(=O)[C@H](CS)N([Si](C)(C)C)[Si](C)(C)C | 1982.3 | Standard non polar | 33892256 | | Cysteinylglycine,3TMS,isomer #7 | C[Si](C)(C)N(CC(=O)O)C(=O)[C@H](CS)N([Si](C)(C)C)[Si](C)(C)C | 2361.9 | Standard polar | 33892256 | | Cysteinylglycine,4TMS,isomer #1 | C[Si](C)(C)OC(=O)CNC(=O)[C@H](CS[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C | 2097.6 | Semi standard non polar | 33892256 | | Cysteinylglycine,4TMS,isomer #1 | C[Si](C)(C)OC(=O)CNC(=O)[C@H](CS[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C | 2143.9 | Standard non polar | 33892256 | | Cysteinylglycine,4TMS,isomer #1 | C[Si](C)(C)OC(=O)CNC(=O)[C@H](CS[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C | 2312.8 | Standard polar | 33892256 | | Cysteinylglycine,4TMS,isomer #2 | C[Si](C)(C)N[C@@H](CS[Si](C)(C)C)C(=O)N(CC(=O)O[Si](C)(C)C)[Si](C)(C)C | 2007.1 | Semi standard non polar | 33892256 | | Cysteinylglycine,4TMS,isomer #2 | C[Si](C)(C)N[C@@H](CS[Si](C)(C)C)C(=O)N(CC(=O)O[Si](C)(C)C)[Si](C)(C)C | 2073.2 | Standard non polar | 33892256 | | Cysteinylglycine,4TMS,isomer #2 | C[Si](C)(C)N[C@@H](CS[Si](C)(C)C)C(=O)N(CC(=O)O[Si](C)(C)C)[Si](C)(C)C | 2243.0 | Standard polar | 33892256 | | Cysteinylglycine,4TMS,isomer #3 | C[Si](C)(C)OC(=O)CN(C(=O)[C@H](CS)N([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2033.3 | Semi standard non polar | 33892256 | | Cysteinylglycine,4TMS,isomer #3 | C[Si](C)(C)OC(=O)CN(C(=O)[C@H](CS)N([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2085.1 | Standard non polar | 33892256 | | Cysteinylglycine,4TMS,isomer #3 | C[Si](C)(C)OC(=O)CN(C(=O)[C@H](CS)N([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2243.9 | Standard polar | 33892256 | | Cysteinylglycine,4TMS,isomer #4 | C[Si](C)(C)SC[C@@H](C(=O)N(CC(=O)O)[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C | 2145.0 | Semi standard non polar | 33892256 | | Cysteinylglycine,4TMS,isomer #4 | C[Si](C)(C)SC[C@@H](C(=O)N(CC(=O)O)[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C | 2174.7 | Standard non polar | 33892256 | | Cysteinylglycine,4TMS,isomer #4 | C[Si](C)(C)SC[C@@H](C(=O)N(CC(=O)O)[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C | 2311.9 | Standard polar | 33892256 | | Cysteinylglycine,5TMS,isomer #1 | C[Si](C)(C)OC(=O)CN(C(=O)[C@H](CS[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2175.5 | Semi standard non polar | 33892256 | | Cysteinylglycine,5TMS,isomer #1 | C[Si](C)(C)OC(=O)CN(C(=O)[C@H](CS[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2207.9 | Standard non polar | 33892256 | | Cysteinylglycine,5TMS,isomer #1 | C[Si](C)(C)OC(=O)CN(C(=O)[C@H](CS[Si](C)(C)C)N([Si](C)(C)C)[Si](C)(C)C)[Si](C)(C)C | 2117.9 | Standard polar | 33892256 | | Cysteinylglycine,1TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CNC(=O)[C@@H](N)CS | 2078.7 | Semi standard non polar | 33892256 | | Cysteinylglycine,1TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)SC[C@H](N)C(=O)NCC(=O)O | 2168.1 | Semi standard non polar | 33892256 | | Cysteinylglycine,1TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)N[C@@H](CS)C(=O)NCC(=O)O | 2124.1 | Semi standard non polar | 33892256 | | Cysteinylglycine,1TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N(CC(=O)O)C(=O)[C@@H](N)CS | 2080.5 | Semi standard non polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CNC(=O)[C@@H](N)CS[Si](C)(C)C(C)(C)C | 2475.1 | Semi standard non polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CNC(=O)[C@@H](N)CS[Si](C)(C)C(C)(C)C | 2351.6 | Standard non polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CNC(=O)[C@@H](N)CS[Si](C)(C)C(C)(C)C | 3142.7 | Standard polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N[C@@H](CS)C(=O)NCC(=O)O[Si](C)(C)C(C)(C)C | 2407.4 | Semi standard non polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N[C@@H](CS)C(=O)NCC(=O)O[Si](C)(C)C(C)(C)C | 2234.1 | Standard non polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N[C@@H](CS)C(=O)NCC(=O)O[Si](C)(C)C(C)(C)C | 2591.7 | Standard polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)CN(C(=O)[C@@H](N)CS)[Si](C)(C)C(C)(C)C | 2296.2 | Semi standard non polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)CN(C(=O)[C@@H](N)CS)[Si](C)(C)C(C)(C)C | 2175.9 | Standard non polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)CN(C(=O)[C@@H](N)CS)[Si](C)(C)C(C)(C)C | 2847.9 | Standard polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N[C@@H](CS[Si](C)(C)C(C)(C)C)C(=O)NCC(=O)O | 2489.2 | Semi standard non polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N[C@@H](CS[Si](C)(C)C(C)(C)C)C(=O)NCC(=O)O | 2380.5 | Standard non polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N[C@@H](CS[Si](C)(C)C(C)(C)C)C(=O)NCC(=O)O | 2828.9 | Standard polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)SC[C@H](N)C(=O)N(CC(=O)O)[Si](C)(C)C(C)(C)C | 2454.6 | Semi standard non polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)SC[C@H](N)C(=O)N(CC(=O)O)[Si](C)(C)C(C)(C)C | 2379.1 | Standard non polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)SC[C@H](N)C(=O)N(CC(=O)O)[Si](C)(C)C(C)(C)C | 2922.2 | Standard polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)N([C@@H](CS)C(=O)NCC(=O)O)[Si](C)(C)C(C)(C)C | 2514.2 | Semi standard non polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)N([C@@H](CS)C(=O)NCC(=O)O)[Si](C)(C)C(C)(C)C | 2306.3 | Standard non polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)N([C@@H](CS)C(=O)NCC(=O)O)[Si](C)(C)C(C)(C)C | 2630.5 | Standard polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)N[C@@H](CS)C(=O)N(CC(=O)O)[Si](C)(C)C(C)(C)C | 2399.9 | Semi standard non polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)N[C@@H](CS)C(=O)N(CC(=O)O)[Si](C)(C)C(C)(C)C | 2227.1 | Standard non polar | 33892256 | | Cysteinylglycine,2TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)N[C@@H](CS)C(=O)N(CC(=O)O)[Si](C)(C)C(C)(C)C | 2603.0 | Standard polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N[C@@H](CS[Si](C)(C)C(C)(C)C)C(=O)NCC(=O)O[Si](C)(C)C(C)(C)C | 2698.7 | Semi standard non polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N[C@@H](CS[Si](C)(C)C(C)(C)C)C(=O)NCC(=O)O[Si](C)(C)C(C)(C)C | 2629.2 | Standard non polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)N[C@@H](CS[Si](C)(C)C(C)(C)C)C(=O)NCC(=O)O[Si](C)(C)C(C)(C)C | 2692.0 | Standard polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)CN(C(=O)[C@@H](N)CS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2665.0 | Semi standard non polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)CN(C(=O)[C@@H](N)CS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2643.6 | Standard non polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)OC(=O)CN(C(=O)[C@@H](N)CS[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2851.6 | Standard polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)CNC(=O)[C@H](CS)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2721.9 | Semi standard non polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)CNC(=O)[C@H](CS)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2614.5 | Standard non polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)CNC(=O)[C@H](CS)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2569.2 | Standard polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N[C@@H](CS)C(=O)N(CC(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2606.4 | Semi standard non polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N[C@@H](CS)C(=O)N(CC(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2530.1 | Standard non polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)N[C@@H](CS)C(=O)N(CC(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2586.1 | Standard polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)SC[C@@H](C(=O)NCC(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2830.2 | Semi standard non polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)SC[C@@H](C(=O)NCC(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2688.0 | Standard non polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #5 | CC(C)(C)[Si](C)(C)SC[C@@H](C(=O)NCC(=O)O)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2731.0 | Standard polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)N[C@@H](CS[Si](C)(C)C(C)(C)C)C(=O)N(CC(=O)O)[Si](C)(C)C(C)(C)C | 2716.9 | Semi standard non polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)N[C@@H](CS[Si](C)(C)C(C)(C)C)C(=O)N(CC(=O)O)[Si](C)(C)C(C)(C)C | 2649.3 | Standard non polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #6 | CC(C)(C)[Si](C)(C)N[C@@H](CS[Si](C)(C)C(C)(C)C)C(=O)N(CC(=O)O)[Si](C)(C)C(C)(C)C | 2682.6 | Standard polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)N(CC(=O)O)C(=O)[C@H](CS)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2704.0 | Semi standard non polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)N(CC(=O)O)C(=O)[C@H](CS)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2594.3 | Standard non polar | 33892256 | | Cysteinylglycine,3TBDMS,isomer #7 | CC(C)(C)[Si](C)(C)N(CC(=O)O)C(=O)[C@H](CS)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2606.0 | Standard polar | 33892256 | | Cysteinylglycine,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CNC(=O)[C@H](CS[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3055.0 | Semi standard non polar | 33892256 | | Cysteinylglycine,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CNC(=O)[C@H](CS[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2903.6 | Standard non polar | 33892256 | | Cysteinylglycine,4TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CNC(=O)[C@H](CS[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2657.1 | Standard polar | 33892256 | | Cysteinylglycine,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N[C@@H](CS[Si](C)(C)C(C)(C)C)C(=O)N(CC(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2924.3 | Semi standard non polar | 33892256 | | Cysteinylglycine,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N[C@@H](CS[Si](C)(C)C(C)(C)C)C(=O)N(CC(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2835.8 | Standard non polar | 33892256 | | Cysteinylglycine,4TBDMS,isomer #2 | CC(C)(C)[Si](C)(C)N[C@@H](CS[Si](C)(C)C(C)(C)C)C(=O)N(CC(=O)O[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2657.7 | Standard polar | 33892256 | | Cysteinylglycine,4TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)CN(C(=O)[C@H](CS)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2918.4 | Semi standard non polar | 33892256 | | Cysteinylglycine,4TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)CN(C(=O)[C@H](CS)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2873.8 | Standard non polar | 33892256 | | Cysteinylglycine,4TBDMS,isomer #3 | CC(C)(C)[Si](C)(C)OC(=O)CN(C(=O)[C@H](CS)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2606.2 | Standard polar | 33892256 | | Cysteinylglycine,4TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)SC[C@@H](C(=O)N(CC(=O)O)[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3033.7 | Semi standard non polar | 33892256 | | Cysteinylglycine,4TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)SC[C@@H](C(=O)N(CC(=O)O)[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2922.4 | Standard non polar | 33892256 | | Cysteinylglycine,4TBDMS,isomer #4 | CC(C)(C)[Si](C)(C)SC[C@@H](C(=O)N(CC(=O)O)[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2672.2 | Standard polar | 33892256 | | Cysteinylglycine,5TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CN(C(=O)[C@H](CS[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3248.9 | Semi standard non polar | 33892256 | | Cysteinylglycine,5TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CN(C(=O)[C@H](CS[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 3098.4 | Standard non polar | 33892256 | | Cysteinylglycine,5TBDMS,isomer #1 | CC(C)(C)[Si](C)(C)OC(=O)CN(C(=O)[C@H](CS[Si](C)(C)C(C)(C)C)N([Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C)[Si](C)(C)C(C)(C)C | 2650.5 | Standard polar | 33892256 |
|
|---|
| GC-MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental GC-MS | GC-MS Spectrum - Cysteinylglycine GC-MS (3 TMS) | splash10-00di-2980000000-043097e8f1f3f47ff51f | 2014-06-16 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Cysteinylglycine GC-EI-TOF (Non-derivatized) | splash10-00di-9440000000-ff8b27f3afc92193d83a | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Experimental GC-MS | GC-MS Spectrum - Cysteinylglycine GC-MS (Non-derivatized) | splash10-00di-2980000000-043097e8f1f3f47ff51f | 2017-09-12 | HMDB team, MONA, MassBank | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Cysteinylglycine GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-9200000000-bae23059b39c80a574a5 | 2016-09-22 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Cysteinylglycine GC-MS (1 TMS) - 70eV, Positive | splash10-006t-8920000000-1b3a6e358d6996916b0e | 2017-10-06 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Cysteinylglycine GC-MS (Non-derivatized) - 70eV, Positive | Not Available | 2021-10-12 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Cysteinylglycine GC-MS (TMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Cysteinylglycine GC-MS (TMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Cysteinylglycine GC-MS (TMS_1_4) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Cysteinylglycine GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Cysteinylglycine GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Cysteinylglycine GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - Cysteinylglycine GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | 2021-11-05 | Wishart Lab | View Spectrum |
MS/MS Spectra| Spectrum Type | Description | Splash Key | Deposition Date | Source | View |
|---|
| Experimental LC-MS/MS | LC-MS/MS Spectrum - Cysteinylglycine LC-ESI-QTOF , positive-QTOF | splash10-004i-9700000000-6fba6043a24b724b037e | 2017-09-14 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Cysteinylglycine 40V, Positive-QTOF | splash10-0a4i-9000000000-50e069074067c9f120bd | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Cysteinylglycine 20V, Positive-QTOF | splash10-004i-9100000000-ab3327c5b13829ea6d35 | 2021-09-20 | HMDB team, MONA | View Spectrum | | Experimental LC-MS/MS | LC-MS/MS Spectrum - Cysteinylglycine 10V, Positive-QTOF | splash10-004i-9700000000-9021340a904a56f317cc | 2021-09-20 | HMDB team, MONA | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteinylglycine 10V, Positive-QTOF | splash10-004i-5900000000-b9a1082799c427caf10b | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteinylglycine 20V, Positive-QTOF | splash10-004i-9200000000-2b99992e97c6ecb4567b | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteinylglycine 40V, Positive-QTOF | splash10-0a6u-9000000000-a8b591f7522fe16637fb | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteinylglycine 10V, Negative-QTOF | splash10-004i-2900000000-0173b5cf52bb61095c3a | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteinylglycine 20V, Negative-QTOF | splash10-00gl-7900000000-f8dc26c3e8b8ec327fc4 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteinylglycine 40V, Negative-QTOF | splash10-0kh9-9100000000-410b0cc1b5e3f850b505 | 2015-09-15 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteinylglycine 10V, Negative-QTOF | splash10-0096-5900000000-55967cd7162376aa6fa2 | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteinylglycine 20V, Negative-QTOF | splash10-00di-9200000000-5aab410f2c44502defce | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteinylglycine 40V, Negative-QTOF | splash10-00di-9000000000-e0ae26ebba3eeba905da | 2021-09-22 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteinylglycine 10V, Positive-QTOF | splash10-056r-9400000000-1878c834f39272d5af42 | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteinylglycine 20V, Positive-QTOF | splash10-056r-9100000000-0899c0bce2addb667ccb | 2021-09-23 | Wishart Lab | View Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - Cysteinylglycine 40V, Positive-QTOF | splash10-0a4i-9000000000-05f619b00f6286d67d21 | 2021-09-23 | Wishart Lab | View Spectrum |
NMR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum | | Predicted 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | 2021-09-29 | Wishart Lab | View Spectrum |
IR Spectra| Spectrum Type | Description | Deposition Date | Source | View |
|---|
| Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M-H]-) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+H]+) | 2023-02-03 | FELIX lab | View Spectrum | | Predicted IR Spectrum | IR Ion Spectrum (Predicted IRIS Spectrum, Adduct: [M+Na]+) | 2023-02-03 | FELIX lab | View Spectrum |
|
|---|
| General References | - Perry TL, Hansen S: Cystinylglycine in plasma: diagnostic relevance for pyroglutamic acidemia, homocystinuria, and phenylketonuria. Clin Chim Acta. 1981 Nov 25;117(1):7-12. [PubMed:7333014 ]
- Bald E, Glowacki R: Analysis of saliva for glutathione and metabolically related thiols by liquid chromatography with ultraviolet detection. Amino Acids. 2005 Jun;28(4):431-3. Epub 2005 May 20. [PubMed:15900404 ]
- Reddy VB, Doss GA, Creighton M, Kochansky CJ, Vincent SH, Franklin RB, Karanam BV: Identification and metabolism of a novel dihydrohydroxy-S-glutathionyl conjugate of a peroxisome proliferator-activated receptor agonist, MK-0767 [(+/-)-5-[(2,4-dioxothiazolidin-5-yl)methyl]-2-methoxy-N-[[(4-trifluoromethyl) phenyl]methyl]benzamide], in rats. Drug Metab Dispos. 2004 Oct;32(10):1154-61. Epub 2004 Jun 30. [PubMed:15229171 ]
- Pastore A, Massoud R, Motti C, Lo Russo A, Fucci G, Cortese C, Federici G: Fully automated assay for total homocysteine, cysteine, cysteinylglycine, glutathione, cysteamine, and 2-mercaptopropionylglycine in plasma and urine. Clin Chem. 1998 Apr;44(4):825-32. [PubMed:9554495 ]
- Mansoor MA, Svardal AM, Ueland PM: Determination of the in vivo redox status of cysteine, cysteinylglycine, homocysteine, and glutathione in human plasma. Anal Biochem. 1992 Feb 1;200(2):218-29. [PubMed:1632485 ]
- Fiskerstrand T, Refsum H, Kvalheim G, Ueland PM: Homocysteine and other thiols in plasma and urine: automated determination and sample stability. Clin Chem. 1993 Feb;39(2):263-71. [PubMed:8432015 ]
- Raijmakers MT, Roes EM, Zusterzeel PL, Steegers EA, Peters WH: Thiol status and antioxidant capacity in women with a history of severe pre-eclampsia. BJOG. 2004 Mar;111(3):207-12. [PubMed:14961880 ]
- Duman BS, Ozturk M, Yilmazeri S, Hatemi H: Thiols, malonaldehyde and total antioxidant status in the Turkish patients with type 2 diabetes mellitus. Tohoku J Exp Med. 2003 Nov;201(3):147-55. [PubMed:14649736 ]
- Van Hove JL, Lazeyras F, Zeisel SH, Bottiglieri T, Hyland K, Charles HC, Gray L, Jaeken J, Kahler SG: One-methyl group metabolism in non-ketotic hyperglycinaemia: mildly elevated cerebrospinal fluid homocysteine levels. J Inherit Metab Dis. 1998 Dec;21(8):799-811. [PubMed:9870205 ]
- Josch C, Sies H, Akerboom TP: Hepatic mercapturic acid formation: involvement of cytosolic cysteinylglycine S-conjugate dipeptidase activity. Biochem Pharmacol. 1998 Sep 15;56(6):763-71. [PubMed:9751082 ]
- Jacobsen DW, Gatautis VJ, Green R, Robinson K, Savon SR, Secic M, Ji J, Otto JM, Taylor LM Jr: Rapid HPLC determination of total homocysteine and other thiols in serum and plasma: sex differences and correlation with cobalamin and folate concentrations in healthy subjects. Clin Chem. 1994 Jun;40(6):873-81. [PubMed:8087981 ]
- Zinellu A, Carru C, Galistu F, Usai MF, Pes GM, Baggio G, Federici G, Deiana L: N-methyl-D-glucamine improves the laser-induced fluorescence capillary electrophoresis performance in the total plasma thiols measurement. Electrophoresis. 2003 Aug;24(16):2796-804. [PubMed:12929176 ]
- Zappacosta B, Manni A, Persichilli S, Scribano D, Minucci A, Lazzaro D, De Sole P, Giardina B: HPLC analysis of some sulphur compounds in saliva: comparison between healthy subjects and periodontopathic patients. Clin Chim Acta. 2003 Dec;338(1-2):57-60. [PubMed:14637266 ]
- Andersson A, Isaksson A, Brattstrom L, Hultberg B: Homocysteine and other thiols determined in plasma by HPLC and thiol-specific postcolumn derivatization. Clin Chem. 1993 Aug;39(8):1590-7. [PubMed:8353942 ]
- Kagedal B, Kallberg M: Cysteinylglycine in urine determined by high-performance liquid chromatography. J Chromatogr. 1984 Jun 8;308:75-82. [PubMed:6746837 ]
- Ueland PM, Mansoor MA, Guttormsen AB, Muller F, Aukrust P, Refsum H, Svardal AM: Reduced, oxidized and protein-bound forms of homocysteine and other aminothiols in plasma comprise the redox thiol status--a possible element of the extracellular antioxidant defense system. J Nutr. 1996 Apr;126(4 Suppl):1281S-4S. [PubMed:8642471 ]
- Ebisch IM, Peters WH, Thomas CM, Wetzels AM, Peer PG, Steegers-Theunissen RP: Homocysteine, glutathione and related thiols affect fertility parameters in the (sub)fertile couple. Hum Reprod. 2006 Jul;21(7):1725-33. Epub 2006 Mar 23. [PubMed:16556671 ]
- Giustarini D, Lorenzini S, Rossi R, Chindamo D, Di Simplicio P, Marcolongo R: Altered thiol pattern in plasma of subjects affected by rheumatoid arthritis. Clin Exp Rheumatol. 2005 Mar-Apr;23(2):205-12. [PubMed:15895891 ]
- Badiou S, Bellet H, Lehmann S, Cristol JP, Jaber S: Elevated plasma cysteinylglycine levels caused by cilastatin-associated antibiotic treatment. Clin Chem Lab Med. 2005;43(3):332-4. [PubMed:15843241 ]
- Morishetti KK, Huang Bde S, Yates JM, Ren J: Gas-phase acidities of cysteine-polyglycine peptides: the effect of the cysteine position. J Am Soc Mass Spectrom. 2010 Apr;21(4):603-14. doi: 10.1016/j.jasms.2009.12.008. Epub 2009 Dec 28. [PubMed:20106677 ]
- Hagos Y, Burckhardt G, Burckhardt BC: Human organic anion transporter OAT1 is not responsible for glutathione transport but mediates transport of glutamate derivatives. Am J Physiol Renal Physiol. 2013 Feb 15;304(4):F403-9. doi: 10.1152/ajprenal.00412.2012. Epub 2012 Dec 19. [PubMed:23255614 ]
- Su T, Xu J, Li Y, Lei L, Zhao L, Yang H, Feng J, Liu G, Ren D: Glutathione-indole-3-acetonitrile is required for camalexin biosynthesis in Arabidopsis thaliana. Plant Cell. 2011 Jan;23(1):364-80. doi: 10.1105/tpc.110.079145. Epub 2011 Jan 14. [PubMed:21239642 ]
- Zinellu A, Sotgia S, Usai MF, Chessa R, Deiana L, Carru C: Thiol redox status evaluation in red blood cells by capillary electrophoresis-laser induced fluorescence detection. Electrophoresis. 2005 May;26(10):1963-8. [PubMed:15812837 ]
- Lau SS, Kuhlman CL, Bratton SB, Monks TJ: Role of hydroquinone-thiol conjugates in benzene-mediated toxicity. Chem Biol Interact. 2010 Mar 19;184(1-2):212-7. doi: 10.1016/j.cbi.2009.12.016. Epub 2009 Dec 23. [PubMed:20034486 ]
- Kedzierska M, Glowacki R, Czernek U, Szydlowska-Pazera K, Potemski P, Piekarski J, Jeziorski A, Olas B: Changes in plasma thiol levels induced by different phases of treatment in breast cancer; the role of commercial extract from black chokeberry. Mol Cell Biochem. 2013 Jan;372(1-2):47-55. doi: 10.1007/s11010-012-1444-2. Epub 2012 Sep 5. [PubMed:22949034 ]
- Steele ML, Fuller S, Maczurek AE, Kersaitis C, Ooi L, Munch G: Chronic inflammation alters production and release of glutathione and related thiols in human U373 astroglial cells. Cell Mol Neurobiol. 2013 Jan;33(1):19-30. doi: 10.1007/s10571-012-9867-6. Epub 2012 Jul 31. [PubMed:22847551 ]
- Carlucci F, Tabucchi A: Capillary electrophoresis in the evaluation of aminothiols in body fluids. J Chromatogr B Analyt Technol Biomed Life Sci. 2009 Oct 15;877(28):3347-57. doi: 10.1016/j.jchromb.2009.07.030. Epub 2009 Jul 24. [PubMed:19651544 ]
- Steele ML, Ooi L, Munch G: Development of a high-performance liquid chromatography method for the simultaneous quantitation of glutathione and related thiols. Anal Biochem. 2012 Oct 1;429(1):45-52. doi: 10.1016/j.ab.2012.06.023. Epub 2012 Jul 1. [PubMed:22759776 ]
- Cappiello M, Alterio V, Amodeo P, Del Corso A, Scaloni A, Pedone C, Moschini R, De Donatis GM, De Simone G, Mura U: Metal ion substitution in the catalytic site greatly affects the binding of sulfhydryl-containing compounds to leucyl aminopeptidase. Biochemistry. 2006 Mar 14;45(10):3226-34. [PubMed:16519517 ]
- Reddy PR, Mohan SK, Rao KS: Ternary zinc(II)-dipeptide complexes for the hydrolytic cleavage of DNA at physiological pH. Chem Biodivers. 2005 May;2(5):672-83. [PubMed:17192010 ]
- Ferin R, Pavao ML, Baptista J: Methodology for a rapid and simultaneous determination of total cysteine, homocysteine, cysteinylglycine and glutathione in plasma by isocratic RP-HPLC. J Chromatogr B Analyt Technol Biomed Life Sci. 2012 Dec 12;911:15-20. doi: 10.1016/j.jchromb.2012.10.022. Epub 2012 Oct 24. [PubMed:23217300 ]
- Hortin GL, Seam N, Hoehn GT: Bound homocysteine, cysteine, and cysteinylglycine distribution between albumin and globulins. Clin Chem. 2006 Dec;52(12):2258-64. Epub 2006 Oct 26. [PubMed:17068168 ]
- Kuzniak E, Kazmierczak A, Wielanek M, Glowacki R, Kornas A: Involvement of salicylic acid, glutathione and protein S-thiolation in plant cell death-mediated defence response of Mesembryanthemum crystallinum against Botrytis cinerea. Plant Physiol Biochem. 2013 Feb;63:30-8. doi: 10.1016/j.plaphy.2012.11.014. Epub 2012 Nov 28. [PubMed:23228550 ]
- Prakash H, Shodai A, Yasui H, Sakurai H, Hirota S: Photocontrol of spatial orientation and DNA cleavage activity of copper(II)-bound dipeptides linked by an azobenzene derivative. Inorg Chem. 2008 Jun 16;47(12):5045-7. doi: 10.1021/ic8007443. Epub 2008 May 14. [PubMed:18476687 ]
- Capone DL, Pardon KH, Cordente AG, Jeffery DW: Identification and quantitation of 3-S-cysteinylglycinehexan-1-ol (Cysgly-3-MH) in Sauvignon blanc grape juice by HPLC-MS/MS. J Agric Food Chem. 2011 Oct 26;59(20):11204-10. doi: 10.1021/jf202543z. Epub 2011 Sep 26. [PubMed:21942856 ]
- Biroccio A, Del Boccio P, Panella M, Bernardini S, Di Ilio C, Gambi D, Stanzione P, Sacchetta P, Bernardi G, Martorana A, Federici G, Stefani A, Urbani A: Differential post-translational modifications of transthyretin in Alzheimer's disease: a study of the cerebral spinal fluid. Proteomics. 2006 Apr;6(7):2305-13. [PubMed:16552785 ]
- Wunschmann J, Krajewski M, Letzel T, Huber EM, Ehrmann A, Grill E, Lendzian KJ: Dissection of glutathione conjugate turnover in yeast. Phytochemistry. 2010 Jan;71(1):54-61. doi: 10.1016/j.phytochem.2009.09.034. Epub 2009 Nov 10. [PubMed:19897216 ]
- Cappiello M, Peroni E, Lepore A, Moschini R, Del Corso A, Balestri F, Mura U: Rapid colorimetric determination of reduced and oxidized glutathione using an end point coupled enzymatic assay. Anal Bioanal Chem. 2013 Feb;405(5):1779-85. doi: 10.1007/s00216-012-6577-3. Epub 2012 Nov 27. [PubMed:23203508 ]
- Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. [PubMed:32033212 ]
|
|---|